Leadership

Rotonya M. Carr, MD
Associate Professor, Medicine
Division Head, UW Gastroenterology
Interim Director, C-LIFE
Research and Innovation
We are a comprehensive multi-disciplinary, self-sustaining Liver Clinical and Translational Research Center.
Key Pillars
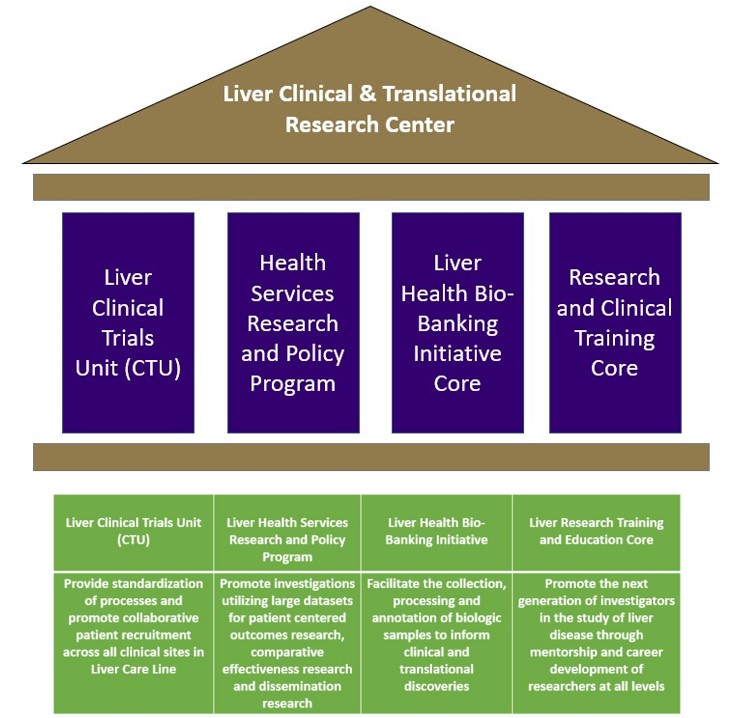
Overview of Key Pillars
Liver Clinical Trials Unit (CTU)
The Center offers funding for targeted research areas and interdisciplinary teams, with a focus on encouraging faculty to channel their effort into collaborations both internally and externally. The Liver CTU provides standardization across all sites and open patient recruitment across all service areas.
Key Features:
- Support for the development and maintenance of a research infrastructure that is a centralized Liver Care Line entity
- Liver Care line centralized web-based patient recruitment and referral
- Funding for a Research Business Operations Manager to assist in hiring, training, and overseeing professional research coordinators
- Access to professional research coordinators, working in a collaborative fashion on clinical trials and funded clinical research
- Dedicated, centralized work-space for all research coordinators and Research Business Operations Manager
Liver Health Services Research and Policy Program
We conduct innovative and high-quality research in comparative effectiveness, health economics and quality of care, patient-centered outcomes, and decision analysis by educating and mentoring future health service researchers.
Key aims:
- Foster Liver Care Line use of large datasets for patient-centered outcomes research and comparative effectiveness research
- Provide training and analytic support for high-quality health services and epidemiologic research
- Acquire and update large clinical & administrative and data sets (UNOS/SRTR, NHANES, NIS, SEER, NHDS, NSQIP, National VA administrative & Clinical, NHIS, BRFSS, NHAMCS)
- Support for Biostatistics Consulting – this service will be based on need
Liver Health Bio-Banking Initiative Core
The Liver Health Bio-Bank was established to collect biological samples from patients with various types of liver disease. The objectives of the Bio-Bank are to stimulate collaboration between clinicians and researchers, to strengthen the training of junior investigators, and to promote multidisciplinary integration to advance translational medicine and improve the health of patients. The goal is to obtain samples that represent patients seen in the clinics of medical centers in Washington and from some of the rarer diseases encountered in these clinics. The ultimate goal is to develop the Bio-Bank as Washington’s resource for clinically annotated human samples that can be used to conduct research that could lead to significant advances in patient care.
Key resources:
- Access to -80 degree freezer and 20-degree freezer capacity with dedicated space for bio-banking
- Access to Lab Technician support for maintenance of samples and cataloging and retrieval
- Lab Technician support for sample processing.
- Access to Centrifuge
- Integrate clinical database and EMR of Liver Tumor Clinic and Liver Care Line to generate real-time clinical annotation
Liver Research and Clinical Training Core
The Center will look to sponsor various programs designed to promote the development of clinical and translational research projects and assist in career development for clinical researchers at all levels.
Programs:
- Innovations in Liver Clinical & Translational Research Pilot Grants
- Trainee Scholar Awards
Benchmarks of Success
Our Goals
To support clinical and translational research
To encourage interdisciplinary research
To promote advanced training
To disseminate research findings
Reaching Our Goals
Supporting clinical and translational research
Activities:
- Encourage external grant proposals using internal grants to fund seed money for pilot studies
- Center Administration helps to develop and submit grant proposals
- Support for Medical Editor for Center members
- Annual grant writing workshop, external speaker for faculty and trainees
- Identify internal and external funding opportunities and communicate with Center faculty
- Become a leading site for clinical trials in Hepatology by assisting faculty in setting up and accruing patients to trials
Evaluation:
- The number of internal grants submitted and funded
- The number of internal grants that develop into grants proposals and funding
- The number of grant workshops and their attendance by faculty and fellows
- The number and quality of assistance provided to Center faculty by the Center Administration
- Increase patient participation in clinical trials
- An increase in journal publications
Encourage interdisciplinary research across departments, colleges, and sites
Activities:
- Encourage interdisciplinary activities using internal funds for speakers and faculty research
- Identify internal and external opportunities and communicate with Center faculty
- Create a searchable website to assist faculty in making collaborative connections and centralizing recruitment
Evaluation:
- The number of internal grants submitted and funded promoting new collaborations that develop into grants proposals and funding
Promoting Advance training in research methods
Activities:
- Center funding of speakers and workshops
- Encourage the funding of statistical support
Evaluation:
- Number of attendees of talks and workshops
- An increase in the number of collaborations and consultants with the statistician, as well as their inclusion in external grant proposals
- An increase in journal publications where statistical data increase the likelihood of publication
Disseminating Research Findings that Address Significant Challenges
Activities:
- Create a website that raises the visibility of the research achievements of the Center, helps publicize funding opportunities, and helps facilities outreach and development.
Evaluation:
- Increased level of awareness of activities within the Center, research, and opportunities by various groups within and outside of the Division of Gastroenterology
CLINICAL TRIAL INFORMATION
If you have a patient* that might be eligible for one of our clinical trials, please send an email to HepTrial@uw.edu with the following information:
- Patient Name and MRN
- Diagnosis
- Any treatment received/provided
- Current Liver Biopsy - yes/no
- Other relevant information
*By submitting the patient's information to C-LIFE, you agree that our research team may contact the patient directly about clinical trials, if appropriate.